 External Stakeholder Panel
External Stakeholder Panel
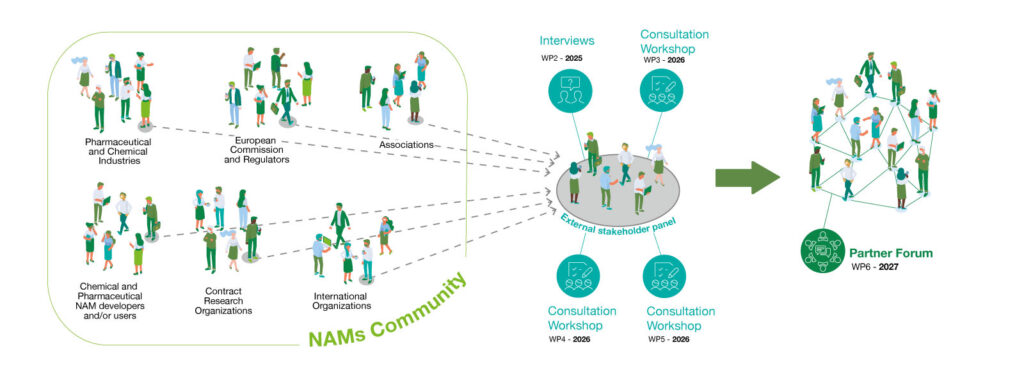
The NAMWISE External Stakeholder Panel is a cornerstone of the project multi-actor approach,
designed to foster inclusive dialogue, knowledge exchange, and consensus-building across the chemical and pharmaceutical sectors.
This panel brings together a diverse group of experts, such as regulators, NAM developers and users (including CROs and RTOs), industry representatives, associations, and civil society organisations, who play a pivotal role in shaping the future of non-animal testing methodologies in Europe.
 Video interview
Video interview
 Purpose & Role
Purpose & Role
Identifying
drivers and obstacles to NAM adoption.
Enhancing
regulatory confidence and legal certainty in NAM-based data.
Providing
feedback on case studies and assessment frameworks.
Supporting
the validation and standardisation of NAMs.
Facilitating
cross-sectoral dialogue and alignment with EU roadmaps and international initiatives.
Members are selected from the extensive networks of NAMWISE partners,
ensuring broad representation and expertise across Europe.
Activities: Panel members actively participate in:
Consultation workshops on case studies and validation strategies.
Scenario modelling for socio-economic impact assessments.
Partner forums to exchange best practices and adopt recommendations.
Feedback loops for refining NAM assessment frameworks and training resources.
These activities are designed to maximise the relevance, applicability, and impact of NAMWISE outputs, while supporting the European Commission’s roadmap to phase out animal testing.

ESP Distribution by Type

ESP Distribution by Sector
ESP Distribution by Countries

 Strategic impact
Strategic impact
By integrating stakeholder insights into every phase of the project, the External Stakeholder Panel helps:
- Build trust and confidence in NAMs among regulators and industry.
- Promote regulatory harmonisation and the “One Substance, One Assessment” approach.
- Accelerate the transition to animal-free safety and efficacy testing.
Ensure that NAMWISE outcomes are scientifically robust, socially accepted, and policy-relevant.