 NAMs Within Integrated Safety & Efficacy evaluation of chemicals and pharmaceuticals
NAMs Within Integrated Safety & Efficacy evaluation of chemicals and pharmaceuticals
The overarching aim of NAMWISE project is to encourage and facilitate the uptake of NAMs (New Approach Methodologies) by providing a defined set of recommendations with concrete guidance and associated training resources on how effectively validate, integrate, and use NAMs for the assessment of the safety and efficacy of chemicals and pharmaceuticals. The NAMWISE project adopts the NAM definition with the maximum leverage for driving the paradigm shift in (eco)toxicology: a definition that does not rely on live non-human vertebrate animals including independently feeding larval forms; and foetal forms of mammals as from the last third of their normal development, and live cephalopods (as defined by the directive 2010/63/EU). The NAMWISE project will explore how, one NAM or a combination of NAMs, can be used to fulfill regulatory requirements for chemicals/pharmaceuticals safety and drug efficacy.
According to this definition the project will focus on the NAM expertise characterizing its consortium: in vitro including organoids and organ-on-chips, and in silico approaches, Adverse Outcome Pathways (AOP) and Integrated Approaches to Testing and Assessment (IATA). In line with existing European initiatives, the project will gather outcomes and identify potential shortcomings, propose solutions and recommendations for the next EU roadmap to phase out regulatory animal testing.
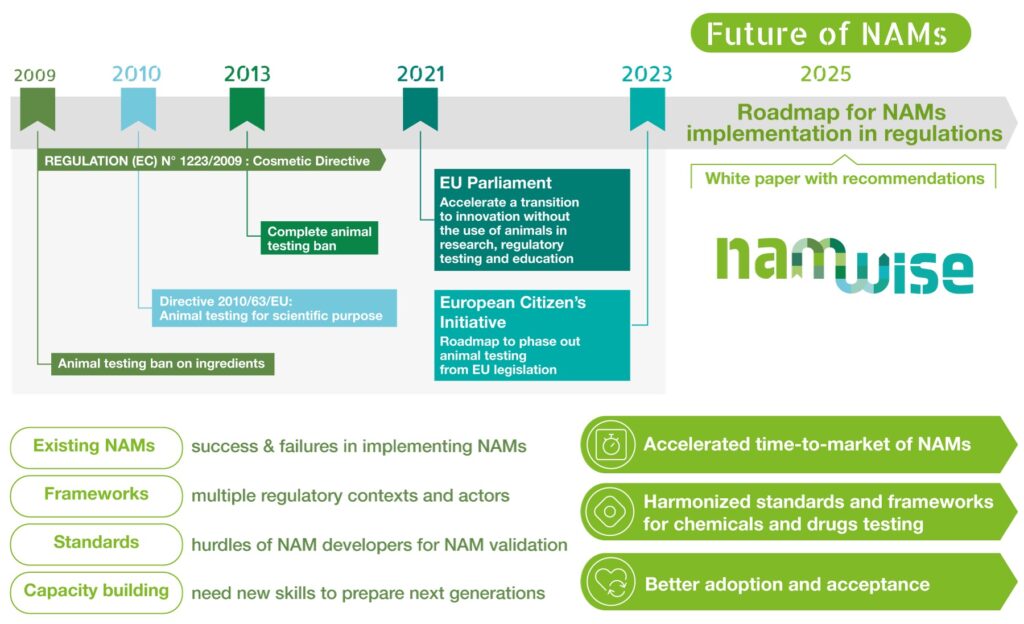
 A Multi-Actor Approach to Advancing NAMs
A Multi-Actor Approach to Advancing NAMs

NAMWISE will implement a multi-actor approach bringing together the NAM value chain i.e.: developers, users, CROs, regulators and stakeholders from both the chemical and pharmaceutical sectors: project activities will be largely based on an iterative dialogue among the stakeholders belonging to the project, and beyond the consortium, to reach a consensus on the most critical aspects regarding NAMs. To ensure successful uptake of NAMs, knowledge, experience and confidence on NAMs and data-based NAM is still lacking among regulators along with inter-agency NAM endorsement and industry is lacking legal certainty when generating safety and health data requested by EU legislation. At a glance, NAMWISE will thus deliver:
- Novel information and knowledge package on NAMs and frameworks, based on the emerging and existing initiatives on NAMs, to contribute to establish knowledge and confidence in NAMs for regulatory assessment of chemicals and pharmaceuticals.
- Robust framework for the validation and use of NAMs for the assessment of the safety and efficacy of chemicals and pharmaceuticals, including solutions for the challenges underpinning the strategy “one substance, one assessment” and recommendations for inter-agencies alignment.
- Set of resources for capacity building and training for CROs and regulators.
- Possibilities for implementing sustainable and long-term activities of the consortium beyond the lifetime of NAMWISE to coordinate NAM activities in Europe.
 Building a European Network for NAMs Innovation
Building a European Network for NAMs Innovation
NAMWISE brings together industry including SMEs, developers, regulators, academia, public institute, consulting companies with complementary expertise in NAMs from both the chemical and pharmaceutical sectors and from various European countries. This network will be key in establishing synergies outside the project to capitalize on and build upon the existing knowledge implemented by ongoing and emerging initiatives on NAMs in chemical and pharmaceutical sectors. In a closing workshop for the target groups the white paper of the project will be presented.

 Key Objectives & Missions
Key Objectives & Missions
- Objective 1: Gather knowledge and information on NAMs, existing and emerging frameworks, initiatives, and trainings, in the chemical and pharmaceutical sectors – WP2
- Objective 2: Assess regulatory NAM implementation and NAM vs animal-based procedures: drivers and obstacles – WP3
- Objective 3: Elaborate concrete guidance and practical examples/case studies on how effectively integrate, interpret and use NAMs for the assessment of the safety & efficacy of chemicals and pharmaceuticals – WP4
- Objective 4: Analyse the requirements for the validation and standardisation of NAMs – WP5
- Objective 5: Communicate and disseminate on NAMs advantages to a large audience and build capacity with dedicated trainings – WP6
Project info
Project ID: 101191595
Coordinated by INERIS
Status: Ongoing project
Funded under: Horizon Europe (HORIZON)
Start date: 1 December 2024
End date :31 May 2027
Overall budget: € 2 049 890,00
EU contribution: € 1 977 390,00
Link to Cordis: https://cordis.europa.eu/project/id/101191595
Discover our differents work packages
WP1
Applying NAMs in case studies on chemical and pharmaceutical risk assessment
WP2
Defining validation and standardisation needs
WP3
Evaluating regulatory implementation, obstacles, and opportunities
WP4
Project management and synergies with other EU initiatives
WP5
Mapping existing knowledge and frameworks related to NAMs
WP6
Communication, dissemination, and capacity building